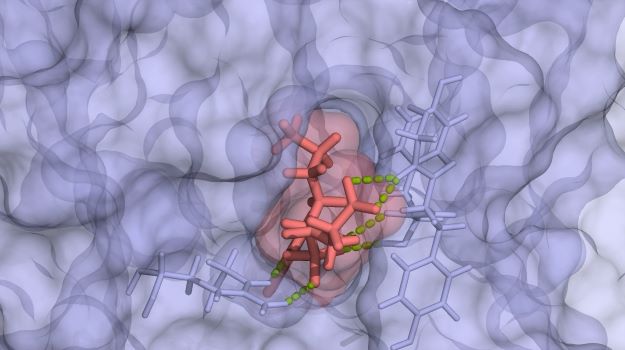
An illustration of bond formation /courtesy of Verseon
Verseon is changing the process of drug development, going beyond artificial intelligence, machine learning and high throughput combinatorial chemistry methods to design drugs atom-by-atom. This physics-based approach enables more perfect binding and, importantly, opens up vast numbers of novel chemical moieties for drug discovery.
The usual drug discovery methods “involve trial and error,” Adityo Prakash, CEO and co-founder of Verseon, told BioSpace. “Even the commonly-used AI screenings rely on known data.” This results in the use of only a tiny percentage of all possible molecules.
For example, he pointed out, “People have estimated the number of truly novel chemical moieties at 1033, yet the industry has made less than 10 million chemical backbones.” Even though the collection of all combinatorial libraries may list hundreds of millions of compounds, those libraries (developed by vendors and drug developers alike) have huge overlaps and contain molecules with high degrees of chemical similarity. This undercuts the ability of high-throughput screening and AI trained on experimental results to find truly novel compounds. The results, he said, are “me-too compounds with small variations. This is like fishing in a droplet of a tidepool compared to the entire ocean!”
Verseon, however, is fishing outside that tidepool in the largely uncharted ocean of chemical space, using molecular physics to accurately predict how new chemical structures will bind to particular proteins.
There are three steps to this process. The first is to create a virtual chemical universe that consists of the rules for chemical reactions. “Others have tried to make virtual libraries,” Prakash said, but didn’t consider the chemical reactions needed to actually make the molecules. “Verseon’s database is a dynamic molecule creation engine. We can point it to any part of the chemical universe we want,” based upon desired features.
“Creating makeable molecules and predicting the synthesis steps is not enough, though,” Prakash said. “The next step is to design and test novel binders for any target in silico.”
Much in silico work is less accurate than desired, so Verseon makes the effort to understand what the binders will look like in three dimensions, how they will flex and twist around a specific chemical bond, where on the protein they will bind, how water affects the interactions and other potential issues.
“If you can model the molecular physics correctly, you can run a vast array of possibilities against any protein target. To do that, we run through many chemotype options, each of which require trillions of calculations, but computing power itself isn’t enough. You also need to know which trillions of calculations to make. That’s where the physics breakthroughs come in,” Prakash said. “If you can do that, you don’t just end up with one or two hits, but several hundred families with completely novel chemicals that nobody else would have found.”
The third step is to make those molecules in the lab, test them in vitro and in vivo, and use the resulting data for AI-driven optimization to achieve highly desirable pharmacokinetic and pharmacodynamic properties for the final candidates.
“If we can do all this right, the paradigm for drug discovery changes,” he said. As Verseon pursues this approach, it is beginning to fill in the map of uncharted drug discovery space. In effect, it is replacing the void of “here be dragons” (as one 16th-century mapmaker warned) with islands of known entities.
“A lot of companies are using AI to validate targets. That’s useful, and we do it, too, but the reality is that we don’t live in a biology-poor world. We live in a biology-rich, chemistry-poor world where the industry is challenged to find truly novel drugs,” Prakash said. “That’s why the pharmaceutical industry’s R&D productivity has dropped over the years.”
What sets Verseon apart is its use of molecular physics modeling to enable the atomic-level engineering of new molecules. This has resulted in a drug discovery program that boasts 14 small molecule drugs in development: six molecules in three cancer programs, and two each for liver disease, cardiovascular disease, diabetic vision loss and hereditary angioedema. “This is a completely disease agnostic platform,” Prakash said.
The cardiovascular program is the most advanced. Verseon is developing first-in-class precision oral anticoagulants (PROACs). The two compounds, VE-1902 and VE-2851, are designed for patients who need lifelong anticoagulant-antiplatelet dual therapy. Currently, he said, “The 51 million patients in the developed world that need long-term anticoagulant and antiplatelet therapy have no safe options.”
Preclinical and Phase I data show VE-1902 significantly reduces blood clots, enables near-normal platelet function and allows near-normal levels of bleeding. Results compared favorably with standard-of-care medications. The second compound, VE-2851, is chemically distinct, with a different mechanism of action. Prakash said it is more potent than VE-1902. “It’s ready for clinical trials.”
Despite having so many molecules in development, Prakash is confident Verseon has the expertise and bandwidth needed to develop them. Since being founded in 2002, “We’ve been systematically building capability. When we decide to target a particular disease area, we bring in the right experts for those programs. Over the years, we’ve learned to optimize our process,” to advance molecules with maximum efficiency.
Prakash, a Caltech graduate, is listed as an inventor on more than 40 patent families. He and cofounder Eniko Fodor are responsible for the development of the technology that enables all video streaming today. The third founder, David Kita, developed one of the first bioinformatics platforms, and thus enabled much of the world’s genomics research for novel gene detection and differential expression profiles. Team members who have held senior leadership positions in big pharma and scientific advisors that include Nobel laureates build out Verseon’s expertise.
Prakash is confident that Verseon’s physics-based approach to designing new binders provides the foundation that allows more accurate drug characterization. Such knowledge alerts designers to consider potential issues early in the drug design process and also opens access to a proverbial ocean of novel chemical types that have never been made.